BOLD ELISA Protocol General Procedure (96-well plate)
BOLD ELISA Protocol General Procedure (96-well plate)
Recommended ELISA plates:
We recommend the use of Maxisorp plates/strips with C bottom wells (e.g., ThermoFisher Cat. No. 430341) for ELISA applications.
Wash buffer
Exazym® Wash Buffer is provided with Exazym® Detection Kits as blister tablets. Exazym® Wash Buffer is prepared by dissolving one tablet in 1 liter of de-ionized water using a laboratory flask or beaker, a magnetic rod and a magnetic stirrer. Allow Exazym® Wash Buffer to reach room temperature.
Capture antibody:
The antibody concentrations should be optimized for each application, but a good starting point is:
-
For a BOLD ELISA being adapted from an existing (standard) ELISA: coat the plate using the same procedure (time, temperature, concentration, etc.) as the standard ELISA.
-
For an ELISA being developed directly using the BOLD technology:
-
We recommend diluting the purified capture antibody to 0.25-6 µg/ml in a coating buffer solution comprising phosphate buffer saline (PBS) at pH 7.4.
-
Add 100 µl of diluted antibody to the wells of an ELISA plate
-
Seal the plate to prevent evaporation. Incubate overnight at 2-8°C.
-
Wash ≥3 x 300µL with PBS-0.05% Tween20 (PBS-T).
Blocking:
For a BOLD ELISA being adapted from an existing (standard) ELISA: blocking can be performed using the same method as the standard ELISA.
For an ELISA being developed directly using the BOLD technology:
-
Block non-specific binding by adding 300 µl of Blocker™Casein (ThermoFisher Cat. No. 37582) per well.
Seal plate and incubate at room temperature for 60 min.
Wash ≥ 3 x 300µL with PBS-T.
NOTE: Our experience is that PBS + 1% casein works well for blocking, but other alternatives which have worked successfully include:
-
PBS with 1% Nonfat Dry Milk (NFDM) (Merck Cat. No. M7409)
-
PBS with 1% BSA (Sigma-Aldrich Cat. No. A7906)
-
Pierce™ Clear Milk Blocking Buffer ThermoFisher Cat. No. 37587)
Standards and Samples:

Add standards and samples diluted in PBS-T +0.1% casein or another appropriate diluent at 100 µl per well.
NOTE: When analyzing plasma and serum samples, an ELISA diluent that blocks heterophilic antibodies may be needed as diluent for samples to prevent false-positive signals. An example of such diluent is Mabtech’s ELISA diluent (Mabtech Cat. No. 3652-D2.)
Seal the plate and incubate it for 60-120 min at room temperature. (Alternatively, if adapting a standard ELISA, use the same incubation time as the standard method.)
Wash 3 x 300µL with PBS-T.
Detection antibody-oligo dT conjugate:
NOTE! Preparation of the antibody-oligo dT conjugate is an offline step which should be performed prior to the start of any BOLD ELISA. Refer to Exazym®ClickChem Conjugation Kit for more detail.
The antibody concentrations should be optimized for each application, but a good starting point is:
- For BOLD ELISA being adapted from an existing (standard) ELISA: Dilute the oligo dT-conjugated detection antibody by a factor of 2-10X as compared to the standard ELISA using PBS-T +0.1% casein as a diluent. (Alternatively, the same diluent as the standard ELISA can be used, as long as it contains an appropriate blocking buffer.)
- For an ELISA being developed directly using the BOLD technology: Dilute the oligo dT-conjugated detection antibody to 0.025-0.5 µg/ml using PBS-T +0.1% casein as a diluent.
NOTE: When analyzing plasma and serum samples, an ELISA diluent that blocks heterophilic antibodies may be needed as diluent for detector antibody to prevent false- positive signals. An example of such diluent is Mabtech’s ELISA diluent (Mabtech Cat. No. 3652-D2.)
Add 100 µl of oligo dT-conjugated detection antibody to each well.
Seal the plate and incubate it for 60 min at room temperature. (Alternatively, if adapting a standard ELISA, use the same incubation time as the standard method.)
Wash ≥ 3 x 300µL with PBS-T.
Polymerase reaction:

Preparation before use – Exazym®Reaction Solution and Exazym®Template mix
Allow Exazym®Reaction Solution and Exazym®Template to thaw and reach room temperature.
Shortly before use, for every mL of Exazym®Reaction Solution add 22 µL of Exazym® Template. For a 96 wells system, 23 µL/well of this mixture is needed per well (2.208 mL in total).
Preparation before use – Exazym®Polymerase Working Solution
Allow the Exazym®Polymerase Buffer to thaw and reach room temperature.
Shortly before use, prepare Exazym®Polymerase Working Solution by diluting Exazym® Polymerase with Exazym Polymerase Buffer to 0.65 U/mL. The concentration of the supplied Exazym®RT Polymerase stock solution may vary between lots and pack sizes. The concentration of the working solution shall be 0.65 U/mL. For a 96 wells system, 77 µL/well is needed per well (7.392 mL in total)
Procedure for polymerization of the hybrid-ladder of DNA:RNA
Add 23 µL/well of Exazym®Reaction Solution/Template mix followed by 77 µL/well of Exazym®Polymerase Working Solution to each well.
Seal the plate and incubate at room temperature for 30 minutes.
Wash ≥ 3 x 300µL with Exazym®Wash Buffer.
DNA:RNA Hybrid Ladder Detection:

Preparations before use – Exazym®Antibody Biotin Working Solution
Allow the Exazym®Antibody Buffer to reach room temperature.
Prepare a 0.3 µg/mL Antibody Biotin Working solution using the Exazym®Antibody Biotin solution supplied with the kit and Exazym®Antibody Buffer. Pre-incubate the antibody Working Solution for 1 hour at room temperature.
Procedure for detection of the DNA:RNA hybrid-ladder
Add 100 µL/well of Exazym®Antibody Biotin Working solution at a concentration of 0.3 µg/mL prepared as described above.
Seal the plate and incubate at room temperature for 30 minutes.
Wash ≥ 3 x 300µL with Exazym®Wash Buffer.
Streptavidin-Horseradish Peroxidase (SA-HRP):
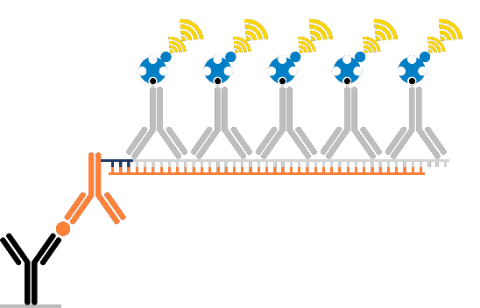
We recommend Streptavidin-HRP conjugate (Bio-techne Cat. No. DY998).
Dilute SA-HRP 1:200 by adding 5 µL SA-HRP per 995 µL PBS-T +0.1% casein. For a 96 well system, 100 µL/well is needed per well (9.6 mL in total).
Add 100 µL/well.
Seal the plate and incubate at room temperature for 30 minutes.
Wash ≥ 6 x 300µL with Exazym®Wash Buffer.
Substrate:
We recommend 1-step Ultra TMB (ThermoFisher Cat. No. 34028) as a substrate.
Equilibrate the TMB to room temperature.
Add 100 µL of the TMB to each well.
Incubate plate at room temperature in the dark 30 minutes
Stop reaction by adding 100 µL of 2M sulfuric acid to each well.
Shake the plate gently and read the optical density of each well at 450nm.
List of buffers and solutions
Phosphate buffer saline (PBS)
80.0 g NaCl, 11.6 g Na2HPO4, 2.0 g KH2PO4, 2.0 g KCl; q.s. to 10 L; pH to 7.4
PBS-0.05% Tween20 (PBS-T)
0.5 ml of Tween®-20 in 1 L PBS.
PBS-T +0.1% casein
Dilute Blocker™Casein 1:10 in PBS-T
Exazym Wash Buffer
Dissolve one tablet in 1 liter of de-ionized water
Exazym®Reaction Solution and Exazym®Template mix
NOTE: This solution should be prepared shortly before use! For every mL of Exazym® Reaction Solution add 22 µL of Exazym®Template. For a 96 wells system, 23 µL/well of this mixture is needed per well (2.208 mL in total).
Exazym®Polymerase Working Solution
NOTE: This solution should be prepared shortly before use! Dilute the Exazym®RT Polymerase with Exazym Polymerase Buffer to 0.65 U/mL. The concentration of the supplied Exazym®RT Polymerase stock solution may vary between lots and pack sizes. The concentration of the working solution shall be 0.65 U/mL. For a 96 wells system, 77 µL/well is needed per well (7.392 mL in total)
Exazym®Antibody Biotin Working Solution
Prepare a 0.3 µg/mL Antibody Biotin Working solution using the Exazym®Antibody Biotin solution supplied with the kit and Exazym®Antibody Buffer. Pre-incubate the antibody Working Solution for 1 hour at room temperature.
SA-HRP Working Solution
Dilute SA-HRP 1:200 by adding 5 µL SA-HRP per 995 µL PBS-T +0.1% casein. For a 96 well system, 100 µL/well is needed per well (9.6 mL in total).
Helpful hints
High backgrounds in blank wells or poor consistency of replicates can be overcome by increasing the stringency of washes and optimizing the concentration of capture and detection antibodies. For example, during washes, the wells can be soaked for ~ 1 minute intervals. Moreover, lower concentrations of detecting antibody or more washes after the detecting antibody stage can reduce background.
A standard curve can be set from row A to row H in the 96-well plate, with the highest concentration in A and the blank in H. Placing the second-highest concentration in row G minimizes the impact of potential edge effects.
For optimal sensitivity, overnight incubation of standards and samples is recommended.
Some BOLD ELISAs benefit from a higher concentration of SA-HRP conjugate. Instead of a 1:200 dilution, a 1:100 dilution can be tested.
Latest update: 2025-10-03
Recommended ELISA plates:
We recommend the use of Maxisorp plates/strips with C bottom wells (e.g., ThermoFisher Cat. No. 430341) for ELISA applications.
Wash buffer
Exazym® Wash Buffer is provided with Exazym® Detection Kits as blister tablets. Exazym® Wash Buffer is prepared by dissolving one tablet in 1 liter of de-ionized water using a laboratory flask or beaker, a magnetic rod and a magnetic stirrer. Allow Exazym® Wash Buffer to reach room temperature.
Capture antibody:

The antibody concentrations should be optimized for each application, but a good starting point is:
-
For a BOLD ELISA being adapted from an existing (standard) ELISA: coat the plate using the same procedure (time, temperature, concentration, etc.) as the standard ELISA.
-
For an ELISA being developed directly using the BOLD technology:
-
We recommend diluting the purified capture antibody to 0.25-6 µg/ml in a coating buffer solution comprising phosphate buffer saline (PBS) at pH 7.4.
-
Add 100 µl of diluted antibody to the wells of an ELISA plate
-
Seal the plate to prevent evaporation. Incubate overnight at 2-8°C.
-
Wash ≥3 x 300µL with PBS-0.05% Tween20 (PBS-T).
-
Blocking:
For a BOLD ELISA being adapted from an existing (standard) ELISA: blocking can be performed using the same method as the standard ELISA.
For an ELISA being developed directly using the BOLD technology:
-
Block non-specific binding by adding 300 µl of Blocker™Casein (ThermoFisher Cat. No. 37582) per well.
Seal plate and incubate at room temperature for 60 min.
Wash ≥ 3 x 300µL with PBS-T.
NOTE: Our experience is that PBS + 1% casein works well for blocking, but other alternatives which have worked successfully include:
-
PBS with 1% Nonfat Dry Milk (NFDM) (Merck Cat. No. M7409)
-
PBS with 1% BSA (Sigma-Aldrich Cat. No. A7906)
-
Pierce™ Clear Milk Blocking Buffer ThermoFisher Cat. No. 37587)
Standards and Samples:

Add standards and samples diluted in PBS-T +0.1% casein or another appropriate diluent at 100 µl per well.
NOTE: When analyzing plasma and serum samples, an ELISA diluent that blocks heterophilic antibodies may be needed as diluent for samples to prevent false-positive signals. An example of such diluent is Mabtech’s ELISA diluent (Mabtech Cat. No. 3652-D2.)
Seal the plate and incubate it for 60-120 min at room temperature. (Alternatively, if adapting a standard ELISA, use the same incubation time as the standard method.)
Wash 3 x 300µL with PBS-T.
Detection antibody-oligo dT conjugate:

NOTE! Preparation of the antibody-oligo dT conjugate is an offline step which should be performed prior to the start of any BOLD ELISA. Refer to Exazym®ClickChem Conjugation Kit for more detail.
The antibody concentrations should be optimized for each application, but a good starting point is:
- For BOLD ELISA being adapted from an existing (standard) ELISA: Dilute the oligo dT-conjugated detection antibody by a factor of 2-10X as compared to the standard ELISA using PBS-T +0.1% casein as a diluent. (Alternatively, the same diluent as the standard ELISA can be used, as long as it contains an appropriate blocking buffer.)
- For an ELISA being developed directly using the BOLD technology: Dilute the oligo dT-conjugated detection antibody to 0.025-0.5 µg/ml using PBS-T +0.1% casein as a diluent.
NOTE: When analyzing plasma and serum samples, an ELISA diluent that blocks heterophilic antibodies may be needed as diluent for detector antibody to prevent false- positive signals. An example of such diluent is Mabtech’s ELISA diluent (Mabtech Cat. No. 3652-D2.)
Add 100 µl of oligo dT-conjugated detection antibody to each well.
Seal the plate and incubate it for 60 min at room temperature. (Alternatively, if adapting a standard ELISA, use the same incubation time as the standard method.)
Wash ≥ 3 x 300µL with PBS-T.
Polymerase reaction:

Preparation before use – Exazym®Reaction Solution and Exazym®Template mix
Allow Exazym®Reaction Solution and Exazym®Template to thaw and reach room temperature.
Shortly before use, for every mL of Exazym®Reaction Solution add 22 µL of Exazym® Template. For a 96 wells system, 23 µL/well of this mixture is needed per well (2.208 mL in total).
Preparation before use – Exazym®Polymerase Working Solution
Allow the Exazym®Polymerase Buffer to thaw and reach room temperature.
Shortly before use, prepare Exazym®Polymerase Working Solution by diluting Exazym® Polymerase with Exazym Polymerase Buffer to 0.65 U/mL. The concentration of the supplied Exazym®RT Polymerase stock solution may vary between lots and pack sizes. The concentration of the working solution shall be 0.65 U/mL. For a 96 wells system, 77 µL/well is needed per well (7.392 mL in total)
Procedure for polymerization of the hybrid-ladder of DNA:RNA
Add 23 µL/well of Exazym®Reaction Solution/Template mix followed by 77 µL/well of Exazym®Polymerase Working Solution to each well.
Seal the plate and incubate at room temperature for 30 minutes.
Wash ≥ 3 x 300µL with Exazym®Wash Buffer.
DNA:RNA Hybrid Ladder Detection:

Preparations before use – Exazym®Antibody Biotin Working Solution
Allow the Exazym®Antibody Buffer to reach room temperature.
Prepare a 0.3 µg/mL Antibody Biotin Working solution using the Exazym®Antibody Biotin solution supplied with the kit and Exazym®Antibody Buffer. Pre-incubate the antibody Working Solution for 1 hour at room temperature.
Procedure for detection of the DNA:RNA hybrid-ladder
Add 100 µL/well of Exazym®Antibody Biotin Working solution at a concentration of 0.3 µg/mL prepared as described above.
Seal the plate and incubate at room temperature for 30 minutes.
Wash ≥ 3 x 300µL with Exazym®Wash Buffer.
Streptavidin-Horseradish Peroxidase (SA-HRP):

We recommend Streptavidin-HRP conjugate (Bio-techne Cat. No. DY998).
Dilute SA-HRP 1:200 by adding 5 µL SA-HRP per 995 µL PBS-T +0.1% casein. For a 96 well system, 100 µL/well is needed per well (9.6 mL in total).
Add 100 µL/well.
Seal the plate and incubate at room temperature for 30 minutes.
Wash ≥ 6 x 300µL with Exazym®Wash Buffer.
Substrate:
We recommend 1-step Ultra TMB (ThermoFisher Cat. No. 34028) as a substrate.
Equilibrate the TMB to room temperature.
Add 100 µL of the TMB to each well.
Incubate plate at room temperature in the dark 30 minutes
Stop reaction by adding 100 µL of 2M sulfuric acid to each well.
Shake the plate gently and read the optical density of each well at 450nm.
List of buffers and solutions
Phosphate buffer saline (PBS)
80.0 g NaCl, 11.6 g Na2HPO4, 2.0 g KH2PO4, 2.0 g KCl; q.s. to 10 L; pH to 7.4
PBS-0.05% Tween20 (PBS-T)
0.5 ml of Tween®-20 in 1 L PBS.
PBS-T +0.1% casein
Dilute Blocker™Casein 1:10 in PBS-T
Exazym Wash Buffer
Dissolve one tablet in 1 liter of de-ionized water
Exazym®Reaction Solution and Exazym®Template mix
NOTE: This solution should be prepared shortly before use! For every mL of Exazym® Reaction Solution add 22 µL of Exazym®Template. For a 96 wells system, 23 µL/well of this mixture is needed per well (2.208 mL in total).
Exazym®Polymerase Working Solution
NOTE: This solution should be prepared shortly before use! Dilute the Exazym®RT Polymerase with Exazym Polymerase Buffer to 0.65 U/mL. The concentration of the supplied Exazym®RT Polymerase stock solution may vary between lots and pack sizes. The concentration of the working solution shall be 0.65 U/mL. For a 96 wells system, 77 µL/well is needed per well (7.392 mL in total)
Exazym®Antibody Biotin Working Solution
Prepare a 0.3 µg/mL Antibody Biotin Working solution using the Exazym®Antibody Biotin solution supplied with the kit and Exazym®Antibody Buffer. Pre-incubate the antibody Working Solution for 1 hour at room temperature.
SA-HRP Working Solution
Dilute SA-HRP 1:200 by adding 5 µL SA-HRP per 995 µL PBS-T +0.1% casein. For a 96 well system, 100 µL/well is needed per well (9.6 mL in total).
Helpful hints
High backgrounds in blank wells or poor consistency of replicates can be overcome by increasing the stringency of washes and optimizing the concentration of capture and detection antibodies. For example, during washes, the wells can be soaked for ~ 1 minute intervals. Moreover, lower concentrations of detecting antibody or more washes after the detecting antibody stage can reduce background.
A standard curve can be set from row A to row H in the 96-well plate, with the highest concentration in A and the blank in H. Placing the second-highest concentration in row G minimizes the impact of potential edge effects.
For optimal sensitivity, overnight incubation of standards and samples is recommended.
Some BOLD ELISAs benefit from a higher concentration of SA-HRP conjugate. Instead of a 1:200 dilution, a 1:100 dilution can be tested.
Latest update: 2025-10-03
Keep in touch
Join our mailing list to receive new information relating to biomarker discovery and detection.